Magnetic Beads (Carboxylated)
$113.30 – $9,818.55
| SKU | OPTIONS | Price | Quantity | ||
|---|---|---|---|---|---|
| MBC-100 | 5 ml, 5% solid | $113.30 | |||
| MBC-200 | 20 ml, 5% solid | $362.56 | |||
| MBC-300 | 100 ml, 5% solid | $1,402.65 | |||
| MBC-400 | 1000 ml, 5% solid | $9,818.55 |
- Description
- Additional information
- Documents
- Q&A
Description
Description:
PuramagTMsup carboxylate modified magnetic beads are monodispersed, colloidally stable superparamagnetic microparticles coated with high density carboxylic acid groups. They have wide range of applications, including nucleic acid isolation, proteomics, and diagnostic immunoassays. The surface carboxylic acid groups are readily available for covalent coupling of proteins, nucleic acids and other amine-containing molecules.
• High binding capacity and low nonspecific binding.
• Highly responsive to an external magnetic field, thereby enabling quick separation from solution.
• Stable in various buffers and detergents and can withstand PCR thermal cycling conditions.
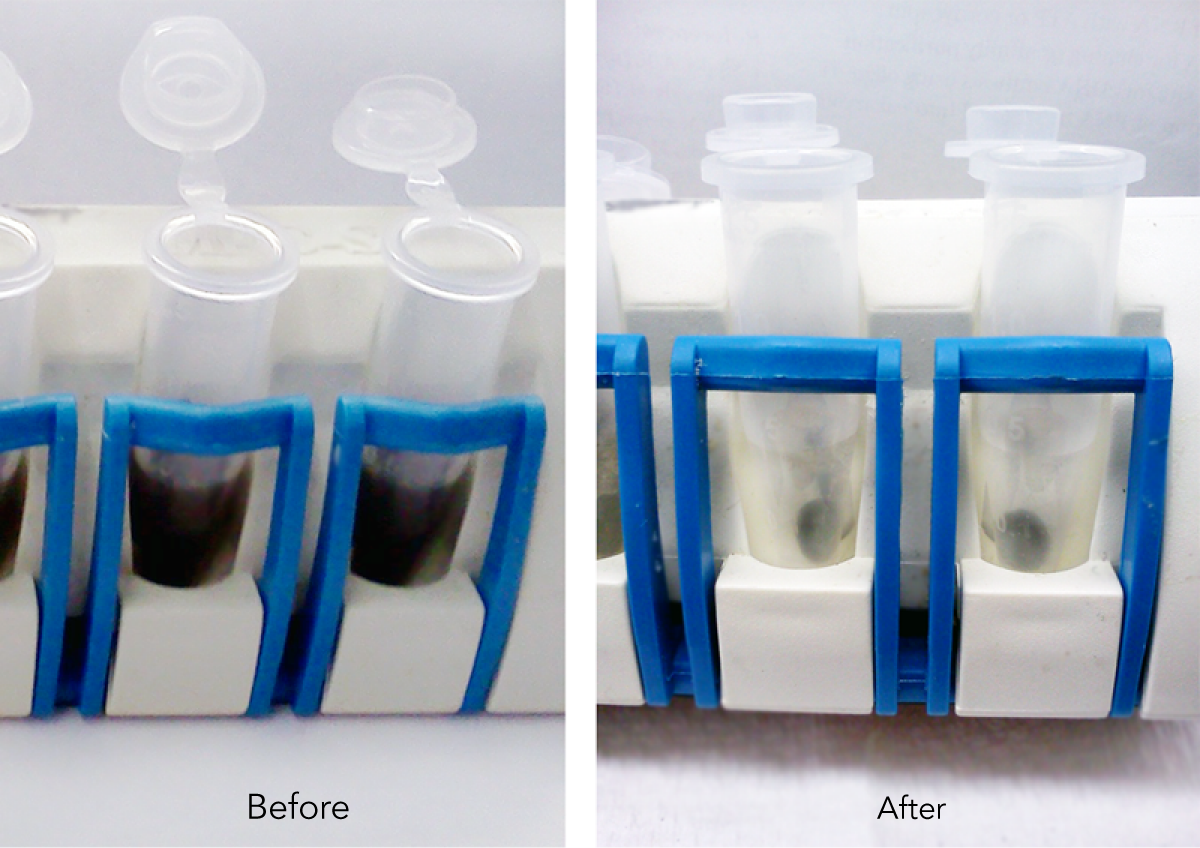
Specification:
Average particle Size: ~ 1 µm
Concentration: 5.0% solid
Carboxyl content: ~ 1.0 meq/g
Magnetite content: ~ 40% (w/w)
Density: ~ 1.4 g/cm3
Recommended storage condition: 2-8 ℃
Protocol
Note:
The following protocol is an example for coupling protein/peptides or other amine-containing ligands to PuramagTM carboxylate modified magnetic beads. We strongly recommend that you optimize the protocol for each individual application. This protocol can be scaled up and down accordingly.
Materials Required
1. McBead™ -6 Magnet Stand or any magnetic separation rack for 1.5-2.0 mL tube.
2. Activation/coupling buffer: 50 mM MES buffer, pH 6.0
3. EDC solution (0.20 M): dissolving 3.8 mg EDC in 100 µl DI-water.
4. Sulfo-NHS solution (0.20 M): add 4.3 mg of Sulfo-NHS to 100 µl of DI-water.
5. Blocking buffer: 1 M Tris buffer, pH 7.5.
6. Wash/storage buffer: 10 mM Tris base, 0.15 M NaCl, 0.1% (w/v) BSA, 1mM EDTA, 0.05% sodium azide, pH 7.5.
Magnetic Beads Preparation
1. Transfer 80 µL of the beads (4 mg) into a 1.5 mL tube, add 500 µl activation/coupling buffer. Mix thoroughly by vortex.
2. Place the tube on the magnet stand for 1-3 minutes and remove the supernatant.
3. Suspend the beads with 500 µL activation/coupling buffer by vortex.
4. Place the tube on the magnet stand for 1-3 minutes and remove the supernatant.
5. Repeat steps 3-4 two times.
6. Suspend the beads in 500 µl of activation/coupling buffer.
One- step coupling
1. Prepare 400 µL of protein solution (0.5-1mg/mL) with DI-water, mix well with the above suspension of washed beads.
2. Add 50 µl of coupling agent (EDC) solution (0.20M) into the tube, mix by vortex.
3. Leave reaction for 2-24 hours at room temperature with gentle rotation.
4. Place the tube on the magnet stand for 1-2 minutes and remove the supernatant.
5. Wash the beads with 1 mL wash/storage buffer three times.
6. Incubate the beads with 1 mL of blocking buffer at room temperature for 1-2 hours.
7. Wash the beads with 1 mL wash/storage buffer three times
8. Suspend the beads with desired volume of wash/storage buffer and store at 2-8 ℃.
Two-step coupling
Note: This protocol is preferred for ligands that contain carboxyl groups or you have only limited amounts of ligand available.
A. Magnetic Beads Activation
1. To 4 mg washed beads suspension in 500 µL activation/coupling buffer add 50 µL EDC solution (0.20M) and 50 µL NHS solution (0.20M), mix well.
2. React ~ 15 minutes with gentle rotation or shaking at room temperature.
3. Place the tube on the magnet stand for 1-2 minutes. Remove the supernatant.
4. Wash the beads with 1 mL activation/coupling buffer three times.
5. Suspend the beads with 500 µL coupling buffer.
B. Coupling of Protein
1. Prepare 400 µl of protein solution (0.5-1mg/mL) with activation/coupling buffer. Mix well with the activated beads by vortex.
2. Leave reaction for 2-24 hour at room temperature with gentle rotation.
3. Place the tube on the magnet stand for 1-2 minutes. Remove the supernatant.
4. Wash the beads with 1 mL activation/coupling buffer three times.
5. Incubate the beads with 1 mL of blocking buffer for 30 minutes at room temperature.
6. Place the tube on the magnet stand for 1-2 minutes. Remove the supernatant.
7. Wash the beads with 1 mL washing/storage buffer three times.
8. Suspend the beads in desired volume of washing/storage buffer and store at 2-8℃.
Additional information
| OPTIONS | 5 ml, 5% solid, 20 ml, 5% solid, 100 ml, 5% solid, 1000 ml, 5% solid |
|---|
MSDS & Certificates
The Q&A for this product will be available soon.
